Smart Fluids
What is a “Smart fluid”?
Smart fluids are non-Newtonian fluids that solidify when external energy in the form of electricity, magnetism, light, etc are applied to them and returns to their original state when the energy is removed. The primary classes of smart fluids are: magneto-rheological fluids (MRFs), electro-rheological fluids (ERFs) and photo-rheological fluids (PRFs).
MRFs can be thought of as dense suspensions of small particles in liquids that solidify into a paste when magnetic field is applied (e.g. iron filings in corn oil produces a primitive MRF). The problem with them is that suspended particles settles and clumps. MRFs were discovered in 1947 and were used in magnetic power clutches in automotive transmissions in the 1950s and in the Apollo service modules in the 1960s. MRFs are now used as resistance brakes in exercise equipment (e.g. step machines and exercise bicycles), shock absorbers, brake, clutch, mount, vibration dampeners, earth quake dampeners, etc.
ERFs behaves as a Bingham plastic when activated and the applied electric field strength defines its yield point. Hence the resistance to motion of the fluid can be controlled by adjusting the applied electric field.
MRFs and ERFs have their specific advantages and disadvantages and they are suitable for different applications. MRFs can resist large forces and they are used in vehicle-suspension systems and structural seismic-vibration damping. MRFs need bulky magnets whereas ERFs require only two electrodes connected by wires to activate the fluid, hence they are attractive for typical oilfield applications. ERFs are researched for the biomedical applications such as prosthetic devices, virtual- reality suit for astronauts, bullet-proof vests, flexible electronics and so on.
Prof. Raghavan had discovered PRFs, a new class of Smart Fluids capable of switching from gel to liquid upon exposure to ultraviolet light. They contain micelles, strings of molecules that spontaneously form in water under the right conditions. Initially, the micelles are long, wormlike chains that tend to get entangled, much like a bowl of spaghetti, making the fluid thick and gel-like. Upon exposure to UV radiation, the micelles length is drastically reduced due to rearrangements of their constituent molecules. The shortened micelles become untangled, and the smart fluid becomes a thin, water-like liquid, with a viscosity (thickness) that is reduced by 4 orders of magnitude (a factor of 10,000).
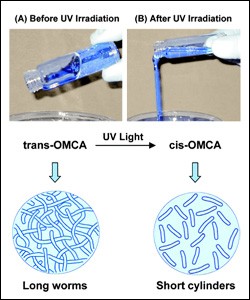
Photo-rheological fluid (PRF)
After many years of research, in 2022, a game changing invention was demonstrated that avoids external agents and stimuli: Self-degrading Organogels based on the self-assembly of molecular gelators. The resulting gels are termed “molecular gels” and they can be formed in water (hydrogels) or organic liquids (organogels). The self-degrading gels degrade without external stimuli or chemicals and the key variable is only the time. For the first time, a class of self-degrading organogels that degrades at a set time period – minutes, hours, or days at a given temperature was demonstrated by Prof. Raghavan. The gels are (a) extremely strong and robust at the initial state, i.e., at time t = 0, the gel modulus G′ is > 10,000 Pa; and (b) they are able to degrade spontaneously into thin sols (G′ ≈ 0) after a pre-determined period of time when left undisturbed.
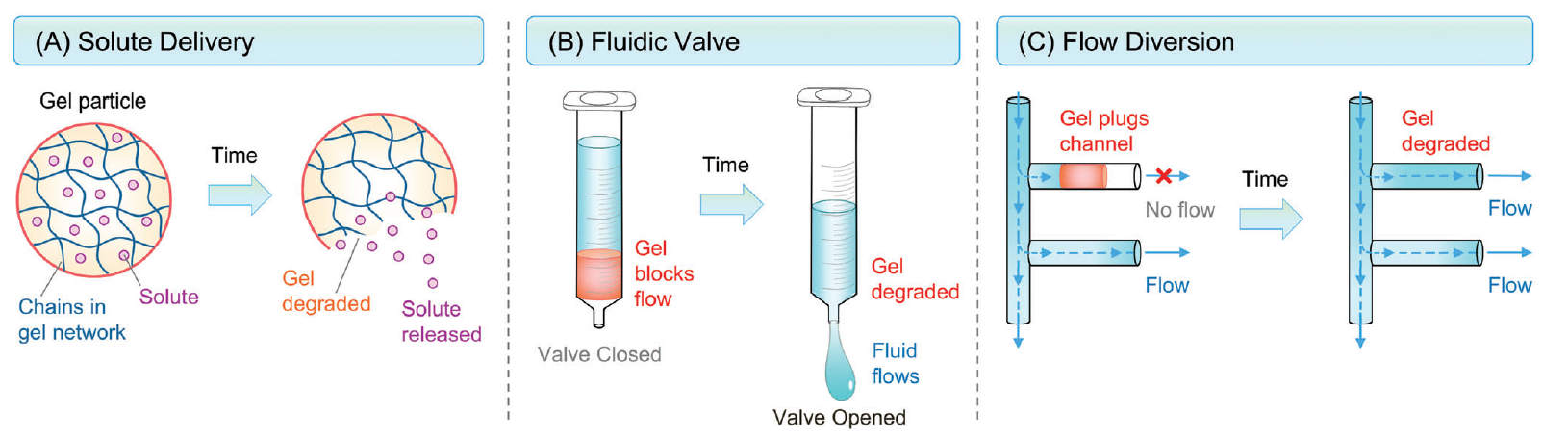
Self-degrading gels and their applications. The gels spontaneously transform into thin sols after a set time
This invention is different from polymers commonly used in the industry. Typical polymeric gel leave residue and long chains of high viscosity remains in the pore space. When molecular gel degrades, the solution becomes a fluid with a very low viscosity. Moreover, because molecular gels form fibrils via non-covalent bonds, the gels will be shear-thinning, i.e., shear will break the bonds and transform the gels into a flowing liquid. Polymer gels held by covalent bonds will splinter into pieces when sheared rather than shear-thin.
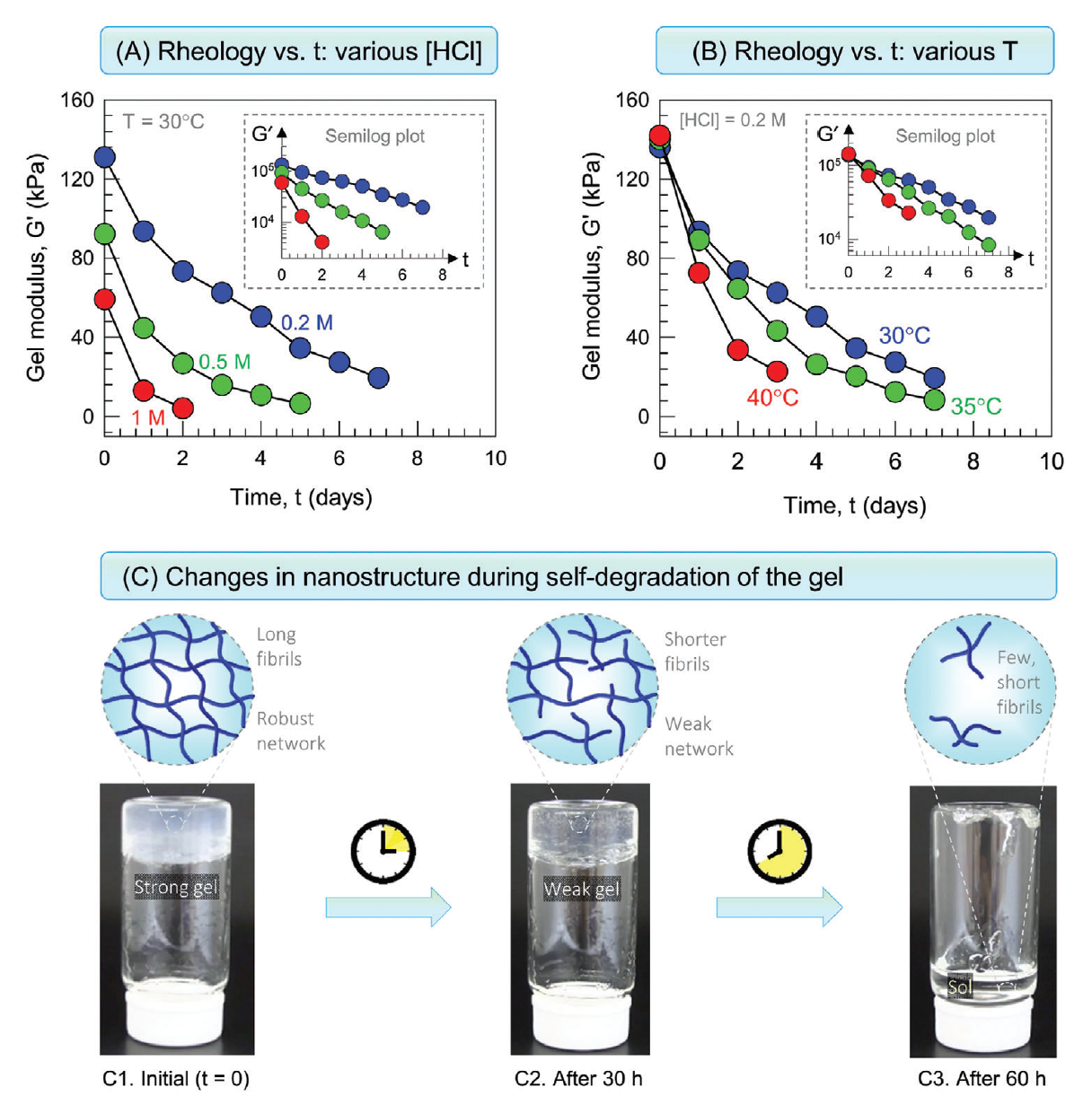
Changes in rheology and nanostructure during the self-degradation of a gel over time.
In 2021, Prof. Raghavan invented and demonstrated Phase-selective aqueous gelation, where an aqueous phase is gelled by adding a component to a coexisting oil phase for the first time. Phase-selective gelation refers to the selective gelation of one phase in an immiscible mixture. While previous research involved a molecular gelator forming nanofibers in the oil phase in an oil/water mixture, for the first time, selective gelation of the water phase in an oil/water mixture (while leaving the oil undisturbed) was achieved
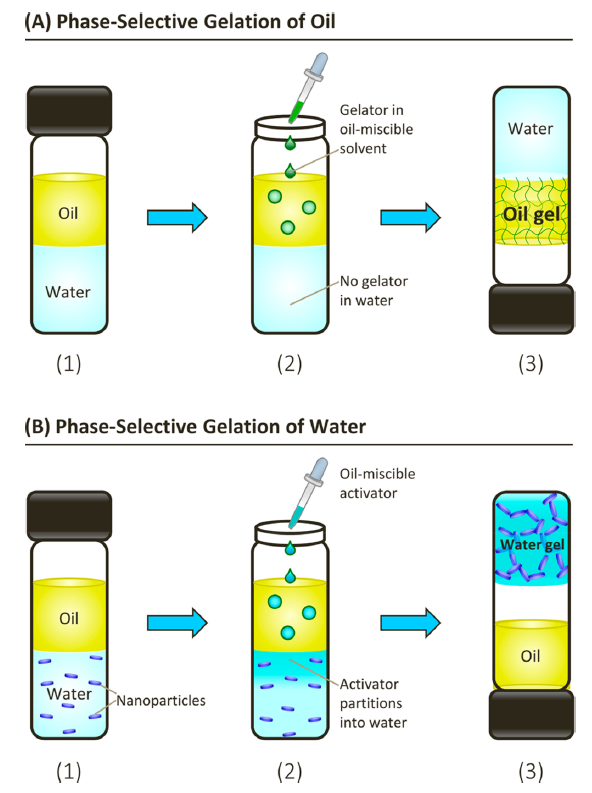
Phase-selective gelation
So, there are many types of Smart fluids and we are able to design and provide operators with an engineering solution depending on the application. Practical considerations at field play an important role in our choice of Smart fluid. We can help you find solutions for some of the toughest challenges in your Oil or Gas field.